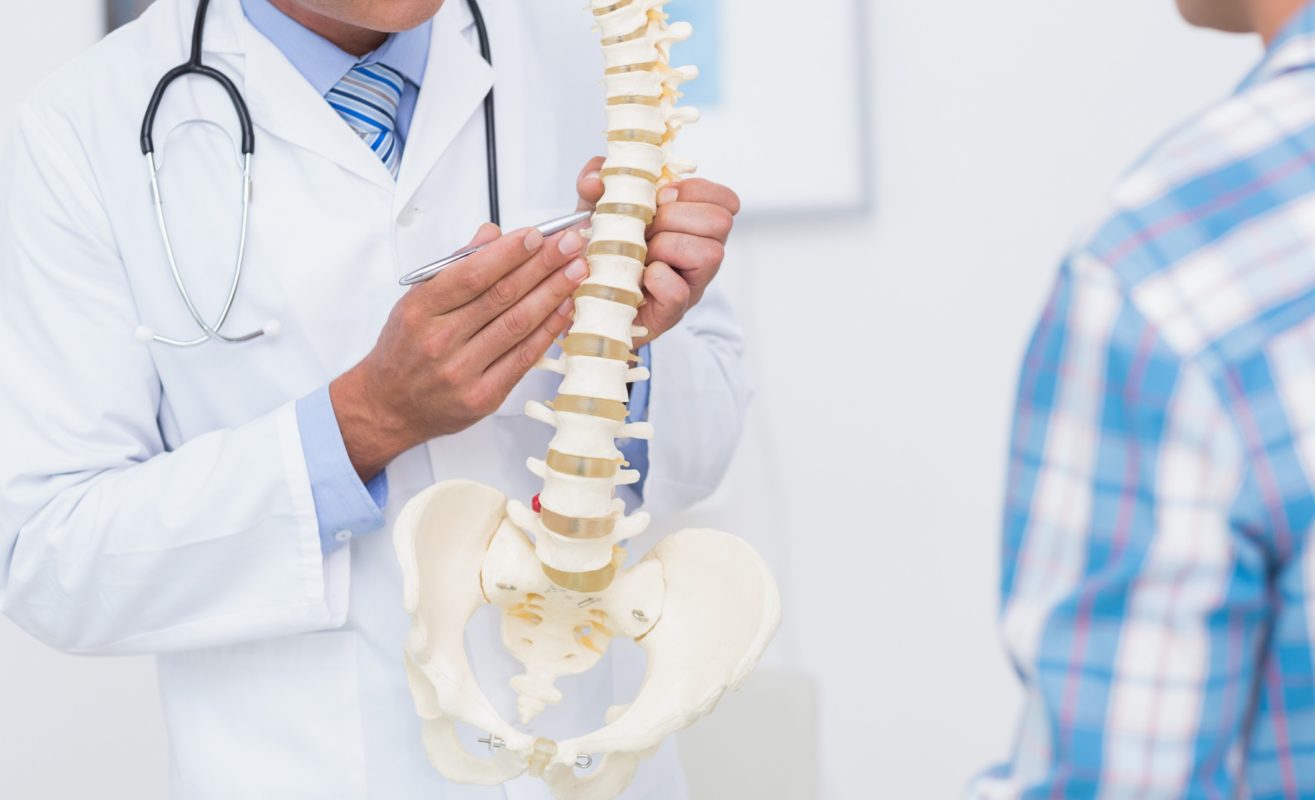
Spinal implants are medical devices surgeons utilize during the surgeries for treating deformity. Deformities treated by spinal implants comprise of disorders like traumatic fractures, spondylolisthesis, kyphosis, degenerative disc disease and scoliosis. The implants steady and reinforce the spine to facilitate fusion.
Spinal Fusion Implants are created through materials which are body-friendly and provide optimal spinal balance. Fusion implants are mutually combined with bone graft. They mainly come in the forms of plates, rods, interbody cages and screws. They are manufactured from diverse types of constituents which include titanium, alloys, steel, stainless steel and the plastics. Titanium implants are light in weight and sturdy, which can be imaged utilizing magnetic resonance imaging.
The implants are made in diverse shapes and sizes which can be curvedor reshaped to be fit as per the surgery requirements. Some of the plates used as implants are less bulky. While some of the screws are coated with the material for stimulating fusion. Growth sparing devices are utilized in surgeries of children as they have not grasped the required skeletal maturity.
Patients must ask questions about the used Spinal Fusion Implants
Patients must take the prime stake about the implants used in their surgery as it is good for their own care. While they are suffering from pain, and the anticipated surgery is a complex operation that cannot be inverted, like spine fusion, the risks go much higher, so it needs to be questioned.
Patients and their families should ask questions to the surgeon related to Spine Fusion that includes:
- What types of spinal implants will be utilized with the fusion?
- Are there any risks involved in the spinal implants used in the fusion?
- Does the hospital has the ownership of the device used for the spinal implant?
- Is the spinal surgery the only option left? Or it has some alternative.
- Are the technologies used in the surgery up to the mark or not?
It is suggested to evade an assumption that the surgeon, the hospital, the medical device company, or the medical device distributor would use the optimum resources and all the required stuffs must be checked personally by the patient and its family members before the surgery.
Spinal implants can be categorized into numerous groups:
- Plates: They are mainly utilized for the cervical spine. Plates are made to conform the spine contour seized by screws which are established into the adjacent vertebrae. When the plate requires adjustment, a contouring tool is used to customize the fit to the patient’s anatomy.
- Rods: Used as original implants in spine. Rods are together utilized with the screws and the hooks, to restrain the spinal levels. They are used to contour the spinal areas to bring them into right alignments. Implant rods are sturdy; however, they have enough elasticity to be shaped to fit with the contours by the surgeon.
- Pedicle Screws: They are specifically made to implant into pedicles of spinal vertebrae, and are used with the special care. They were utilized particularly in lumbar spine, however with technology progressions they are now also utilized in the thoracic spine in the surgery. Screws offer powerful anchorage points wherein rods can be fitted.
- Hooks: They are utilized with the rods implants for anchoring them with the vertebrae.
- Cages: They are fitted amid the vertebrae’s. They are generally made in small shapes and are hollow devices along with the perforated walls. Bone grafts are most often packed about the cages to support the growth in the bones amongst the adjacent vertebrae’s. Cages are utilized mainly to restore lost disc height resultant through a collapsed disc. They are specifically used to relieve pressure on the required nerve roots.
Future Advancements in development of Implants
Scientists and surgeons across the globe are continuously developing improved spinal fusion implants for better surgical results, not only while the surgery but also after it. There are improvements seen in all the surgical and post-surgical departments that has empowered surgeons to bring successful results. A substantial work is going on creating bio-resorbable implants which can be utilized to facilitate fusion. Very few bio-resorbable implants are accessible today, however, their development is a key step taken towards the medical success.
Steps taken for the recovery after Surgery
One needs to consult the surgeon about the recovery plans after the surgery. It is key to work on their instructions sensibly from recovering along the surgery for increasing the chances of fruitful medical results.
Working on the lower back pain is crucial after surgery. The recovery depends how sincerely you work with the physical therapist and how you work out with the suggested exercises by the surgeon. Frequent visits to the surgeon will be required for recovery sessions.
Daily Living after Surgery
After the healing and when you are back to regular activities, you would possibly not feel the spinal implant. The spinal implants are manufactured from metals like titanium, so they can occasionally source problems with the metal detectors. However, your surgeon can provide you a card that can be showed while the checking of metal detections. Using this identification card, you can show the required surgery details while the checking.
Conclusion
There has been a significant advancement seen in the progress of using and manufacturing of spinal fusion implants along with the operated surgeries in the last few years. The outcomes are healthier treatments for the patients. Specific implants are being in research and manufacturing to treat the exact needs of individual patients.
Uteshiya Medicare is an Orthopedic & Trauma implants manufacturers & Suppliers company approved by FDA and ISO 13485:2003. We are India based Orthopedic & Trauma implants manufacturer and supplier. It is managed by a very strong and sound technical legacy set by the Company Directors who have crystal clear foresight and a keen vision with a rich experience and technology savvy background and apt qualification. The company is set to manufacture and produce excellent prime quality Import Substitute Trauma Implants with keen focus on superior service and customer based design and technical solutions. For more details about our products please click here