The titanium mesh cage’s adaptability has made it possible to use it in a wide range of spine surgeries, which has led to its introduction into the medical profession.
Restoring damaged spines has been a common practice using Titanium Mesh Cages since their creation in 1986. In order to use these cages as structural devices that include autologous local bone or iliac crest bone transplant, it is not required to harvest large structural bone grafts.
Sizes of Mesh Cage Titanium Spinal Implants
The finest quality titanium is generally used to manufacture Titanium Mesh cages. Titanium possesses remarkable biocompatibility, strength, and durability.
The fusion site may be seen clearly after surgery due to titanium’s radiolucent nature, which does not interfere with X-rays or other types of imaging.
Titanium mesh cages are available in a range of sizes, including both length and diameter.
| Diameters | Lengths |
| 10mm, 12mm, 14mm, 16mm, 18mm, 20mm, 22mm, 24mm and 26mm | 60mm, 70mm, 80mm, 90mm and 100mm |
Applications of Titanium Mesh Cages.
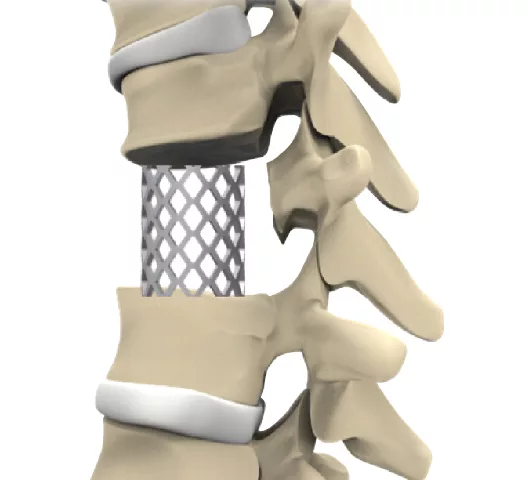
Discs between vertebrae can become damaged or worn over time; spinal fusion surgery involves removing these discs and then filling the area with a bone graft.
The next step is to insert the titanium mesh cage into the disc area so it may function as a spacer or support structure.
It helps to keep the space between the vertebrae intact, promotes the fusion of nearby vertebrae, and returns the spine to its normal height and position.
In order to accommodate bone transplant material, the titanium mesh cage is usually perforated or has pores. Therefore, stimulating bone formation within and surrounding the cage aids in the fusion process.
The cage eventually combines with the surrounding bone as new bone tissue forms around it, providing support and stability to the unified spinal section.
Benefits of Mesh Cage Titanium Spinal Implants.
- When you don’t need to have extensive bone grafts, Mesh Cage Titanium Spinal Implants greatly lower the death rate at donation sites.
- The implants support the structure without the need for big bone grafts, so they are stable without the problems that come with other ways.
- Titanium mesh plates support the anterior spine right away, making it more stable after surgery and speeding up the healing process.
- By using cancellous bone, the implants make it possible for a less invasive autograft harvest. This means that the patients are less affected, and they don’t have to go through long bone transplant treatments.
- A less invasive way to do ACDF is with mesh cage titanium implants, which make the operation easier by creating a small cerebral window on the anterior iliac crest.
- The process for autograft harvest is minimally invasive, requiring only a 1-inch cut. This helps the patient heal faster and feel less pain after surgery.
- By filling the cut area with demineralized bone matrix or cancellous allograft, the implants make it possible to rebuild the iliac crest donor site. This reduces pain and other potential challenges.
- Cages with Mesh Titanium implants are flexible because they let you use removed spinal bodies to fill cages for repairs after a corpectomy. The cortical window method lets you collect more cancellous autografts if you need to.
Behind the Scenes: Manufacturing Process
There are many significant steps involved in making Mesh Cage Titanium spinal implants:
- The biocompatibility and robustness of medical-grade titanium make it an excellent material choice.
- During the design phase, you and the surgeons and biomedical engineers will work together to construct a mesh cage that meets all of the load-bearing and anatomical requirements.
- Obtain titanium sheets or rods for the raw material preparation. The mesh cage may be made by precisely machining titanium to the appropriate specifications.
- For the formation of the mesh, it is necessary to use sophisticated machining processes such as wire EDM or laser cutting. Make sure the design is accurate so it can sustain the structure and let bone grow in.
- Acid etching, anodization, and coatings are surface treatments that can be used to improve biocompatibility and osseointegration.
- Ensure compliance with regulatory requirements by implementing strict techniques at each level for quality control. Verify for correct dimensions, smoothness of surface, and structural integrity.
- Mesh cages can be sterilized using gamma irradiation or ethylene oxide gas to eliminate any possibility of microbes.
- A sterilized mesh cage with all relevant product information and regulatory-appropriate labels should be packaged in a sterile setting.
- Regulatory requirements will dictate the method of distribution for the mesh cages to be used in healthcare institutions or by distributors.
- During spinal surgery, surgeons choose a mesh cage that is the right size for the patient. To provide the spine the support it needs, implant the mesh cage where it needs to be.
- Collaboration with healthcare providers to observe patients after implantation and collect feedback on the implant’s effectiveness constitutes post-implant follow-up.
What are the risk factors for Subsidence of Titanium Mesh Cages?
There are a number of factors that could affect the stability and integration of the Mesh Cage Titanium spinal implants into the spinal section, which increases the risk of subsidence. Some of these criteria are:
- The use of insufficient or poor-quality bone graft may lead to inadequate support for the mesh cage, increasing the risk of subsidence.
- Incorrect placement or insufficient fixation during surgery can contribute to the inadequate stability of the mesh cage, leading to subsidence.
- Choosing a mesh cage that does not properly fit the anatomical dimensions may result in uneven load distribution, increasing the likelihood of subsidence.
- Individual variations in bone density, health, and lifestyle can affect the fusion process and contribute to the risk of subsidence.
- Patients with compromised bone quality, such as osteoporosis, may experience reduced bone density and strength, increasing the susceptibility to subsidence.
- Inadequate contact between the mesh cage and vertebral endplates may hinder proper load transmission, potentially leading to subsidence.
- Incomplete or delayed osseointegration between the mesh cage and surrounding bone can compromise stability, contributing to the risk of subsidence.
- Factors such as excessive postoperative activity or insufficient postoperative immobilization may impact the healing process and increase the risk of subsidence.
- The presence of infections or an inflammatory response around the implant site can compromise the bone healing process and contribute to subsidence risk.
- Abnormal mechanical forces or excessive stress on the spinal segment can lead to increased pressure on the mesh cage, potentially causing subsidence.
- Lack of proper postoperative monitoring and follow-up assessments may result in delayed identification and intervention for subsidence issues.
Are MRIs safe to use with titanium mesh?
The MRI magnetic field has no effect on titanium because it is a paramagnetic substance. Patients with implants can safely have magnetic resonance imaging (MRI) scans since the risk of implant-related problems is minimal. But the craniofacial titanium plates are really alloys. The influences of magnetic resonance imaging (MRI) are proportional to the ratio of the alloy’s components, so more accurate studies are required.
Wrapping It Up
The growth and uses of the Mesh Cage Titanium Spinal Implants highlight a dedication to improving patient outcomes and quality of life through the advancement of medical solutions. It continues to be a durable spinal implant.
